Thermodynamic Terminology and Definitions
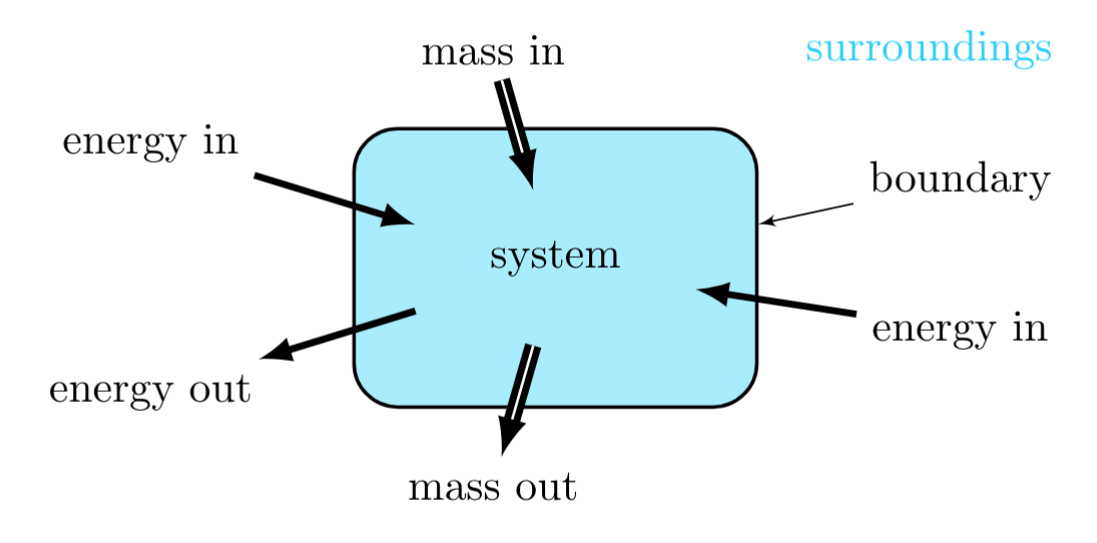
System, boundaries and surroundings
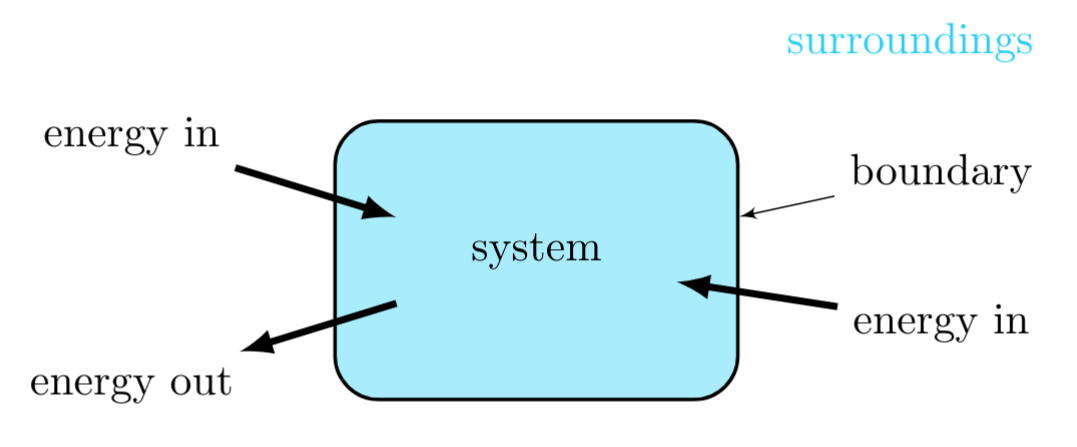
Energy and matter flow between a thermodynamic system and its surroundings that are separated by a boundary, lead to changes its internal state. An increase in its mass results when the matter flowing into the system is greater than that flowing out of the system. An increase in its internal energy results when the energy flowing into the system is greater than that flowing out of the system. When the flow rates into and out of the system are equal, the system is in a steady state.
Steady-state:
When the flow rates into and out of the system are equal, the system is in a steady state
Transient:
The state of a thermodynamic system is transient if it is not in steady state.
Types of Systems: Closed, Open, and Isolated Systems
Closed System:
Mass cannot flow in or out of the system, therefore energy is transferred via only heat or work.
Open System:
An open system can exchange both energy and matter with its surroundings.
Isolated System:
A system is isolated from its environment, meaning neither matter nor energy may flow in or out of the system.
Typical Units in SI and English Systems
Typical Units in SI and English Systems: Dimensions
| Dimension | SI | US |
|---|---|---|
| Length | meter (m) | foot (ft) |
| Mass | kilogram (kg) | pound mass (lb) |
| Time | second (s) | second (s) |
Typical Units in SI and English Systems: State properties
| State property | SI | US |
|---|---|---|
| Pressure | 1 Pa = N/m\( ^2 \) = J/m\( ^3 \) | |
| Volume | m\( ^3 \) | ft\( ^3 \) |
| Temperature | degrees Celcius (\( ^{\circ} \)C) | degrees Fahrenheit (\( ^{\circ} \)F) |
| Absolute Temperature | Kelvin (K) | degree Rankine (\( ^{\circ} \)R) |
| Internal Energy, Enthalpy | Joules (J) | British thermal Unit (Btu) |
| Newton-meter (N\( \cdot \)m) | Foot-pound force (ft\( \cdot \)lbf) | |
| Entropy | (J/K) | Btu/\( ^{\circ} \)R |
Useful Conversions
Pressure
Temperature
Energy
Useful Constants
Gravity
Universal Gas Constant, \( \overline{R} \)
Molecular weights \( M \) of common substances:
Note: (1 g/mol = 1 kg/kmol = 1 lb/lbmol): \( R= \frac{\overline{R}}{M} \)
| Substance | Molecular weight (\(g/mol\)) |
|---|---|
| Air | 28.97 |
| Carbon dioxide CO\( _2 \) | 44.01 |
| Ethane C\( _2 \)H\( _6 \) | 30.07 |
| Hydrogen H\( _2 \) | 2.01 |
| Methane CH\( _4 \) | 16.04 |
| Nitrogen N\( _2 \) | 28.01 |
| Oxygen O\( _2 \) | 32.00 |
| Refrigerant 22 | 86.48 |
| Refrigerant 134a | 102.3 |
| Water H\( _2 \)O | 18.015 |
Helpful Math Expressions
Integrals:
Natural Logs:
Mass and Mass Flow
As matter flows in and out of a thermodynamic system, the amount of mass in the system at time \( t \) by is defined by \( m(t) \), then
refers to 'the rate of change of the mass of the system.' The sums on the right add up all mass flows into and out of the system, respectively. If this quantity is positive, then the mass of the system is increasing. If it is negative, the mass of the system must be decreasing, as long as there is still mass left inside the system.
If these flow rates are known functions of time, then this equation implies that
where \( m(0) \) is the initial value of the mass in the system at time \( t=0 \). Integrating Eqn. MassBalanceInt with respect to time yields.
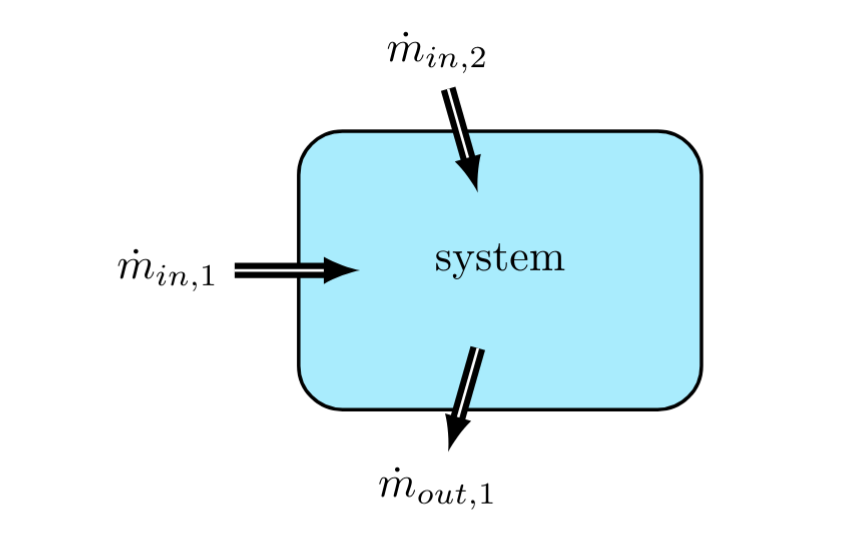
Given the thermodynamic system shown in Figure 2, one can write the rate of change of mass of the system equals the following quantity,
Volume and Volume Flow
The volume \( V \) of a system is an extensive parameter for describing its thermodynamic state. The specific volume \( v \), an intensive property, is the system's volume per unit mass. Volume is a function of state and is interdependent with other thermodynamic properties such as pressure and temperature.
Specific Volume
Specific volume is the volume per unit mass
Volume Flow
As mass flows into a system, energy increases by the addition of both the internal energy of the additional mass and the \( pV \) work done by the volume occupied by the mass on the system as it enters, and vice versa.
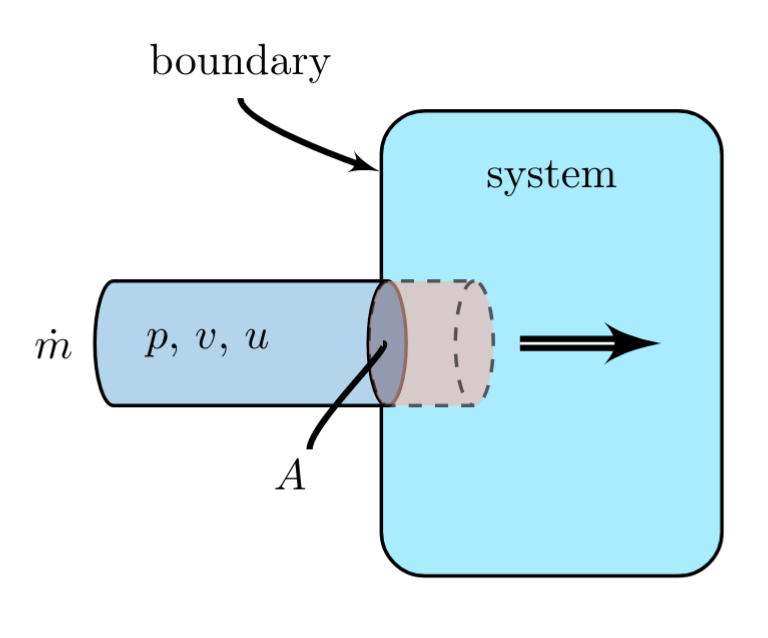
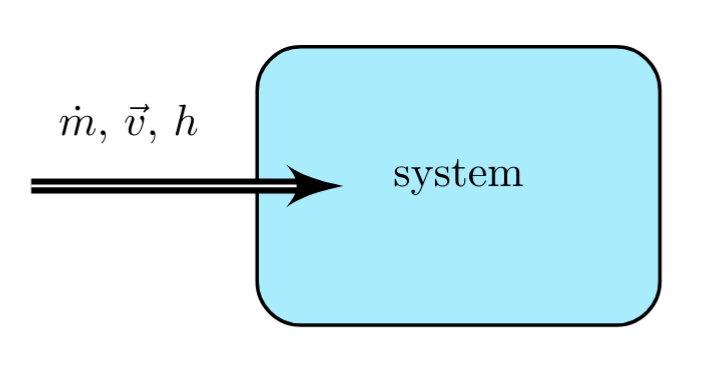
A mass flow having rate \( \dot{m} \) with cross-sectional area, \( A \), slowly enters a system. The mass is at a pressure \( p \), has a specific volume \( v \), and has a specific internal energy, \( u \), relative to some reference state.
The mass flows into the system, over a given time interval \( \Delta t \), increasing the internal energy of the system as it enters, by:
The volume occupied by the portion of the stream entering a system is defined by,
This volume is pushed at constant pressure, \( p \), into the system, is doing \( pV \) work on the system, which will be described further in section \(pV\) work.
Internal Energy
The internal energy, \( U \), of a thermodynamic system relative to some reference state \( U_0 \) is the energy required to set up the positions and motions of the atoms and molecules within the system from their positions and motions in the reference state. When the system is in its reference state, its relative internal energy equals \( 0 \).
The addition of energy to a system in its reference state will result in the system having a positive relative internal energy. The removal of energy from a system in its reference state will result in the system having a negative relative internal energy. Eqn.MassBalanceInt2.
Internal Energy Contributions
The internal energy (\( U \)) of a system is affected by the energetic contributions from heat flow (\( Q \)) in and out of a system and work (\( W \)) done by or on a system.
Sign Convention:
These energetic changes affect thermodynamic systems as follows:
- When heat is transferred to the system, the system energy increases (positive heat transfer).
- When the system does work on its surroundings, the system energy decreases (negative sign in front of work done by system).
- When the system transfers heat to the surroundings, the system energy decreases (negative heat transfer).
- When work is done on to the system, the system energy increases (positive sign in front of work done on system).
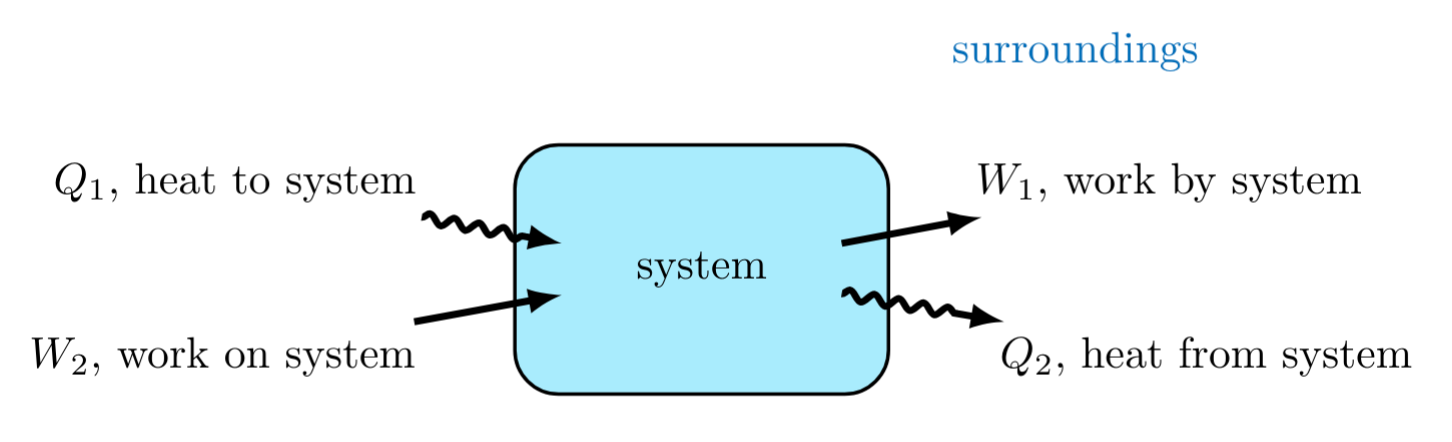
Given the system shown in Figure 4, the net work done by the system is \( W_1-W_2 \). The net heat transferred to the system is \( Q_1-Q_2 \). The change in system energy is
Potential Energy
A system may find itself subject to a conservative force in a position that differs from a reference position. In this scenario, work has been done against a conservative force, such as changing the height, \( h \), to change the potential energy.
Thus the change in the gravitational potential energy of the system.
Kinetic Energy
A system may find itself in motion with respect to a reference frame. In this first case, work has been done to
accelerate the system to its velocity. Work done is defined by forces acting over a distance, and work \( W_{\text{KE}} \) can be done to accelerate the system to its velocity,
Force is determined from Newton's 2nd law:
This results in the following relationship for work done via kinetic energy change (\( W_{\text{KE}} \))
Thus the change in kinetic energy of the system.
Enthalpy
Enthalpy is a state function that is the sum of a system's internal energy \( \mu \) and the product of its pressure and volume \( pv \).
Relative Specific Enthalpy
The sum \( u+pv \) appears frequently in thermodynamic discussion so it is conveniently defined as relative specific enthalpy \( h \), consisting of the same atomic and molecular kinetic and potential energies as specific internal energy \( u \), but with the addition of flow work, \( pv \)