Energy Transfers
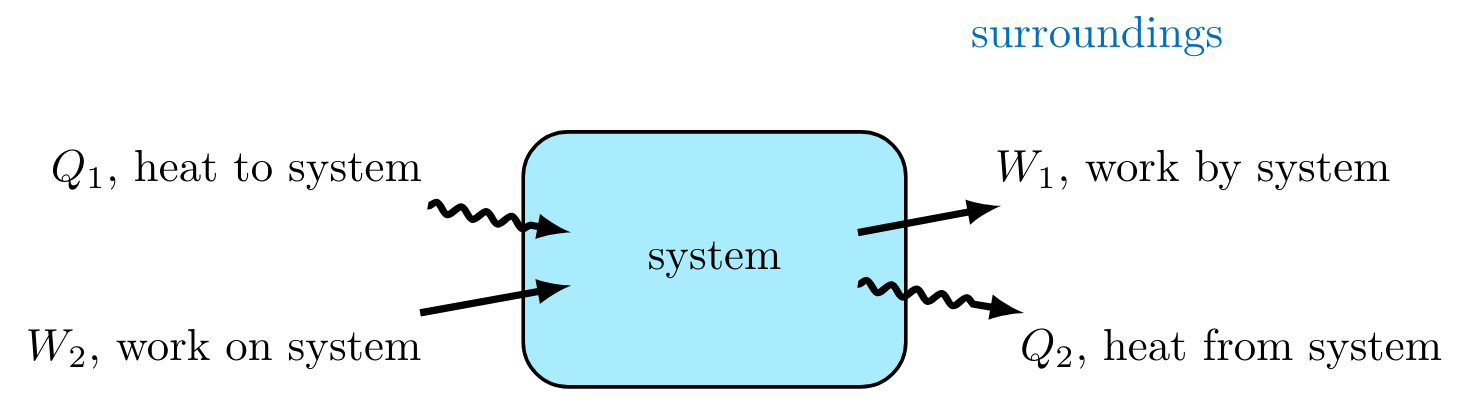
When mass does not flow into or out of a system, the energy of the system changes in only two ways, through the loss or addition of work and/or heat.
The internal energy (\( U \)) of a system is affected by the energetic contributions from heat flow (\( Q \)) in and out of a system and work (\( W \))done by or on a system.
Heat
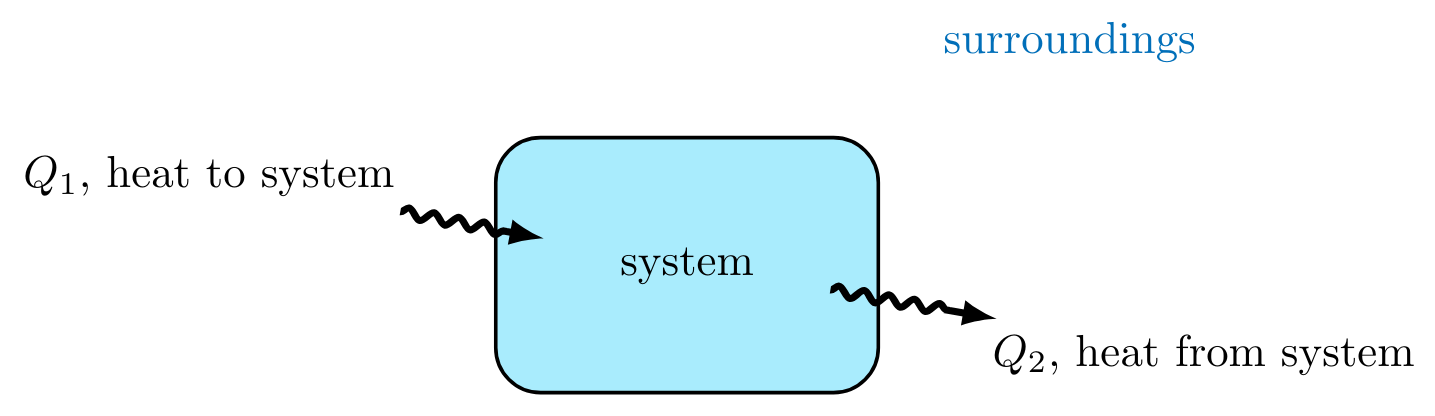
In thermodynamics, heat \( Q \) is the thermal energy transferred between systems due to a temperature difference. Heat can both flow into and out of a system as shown in Figure 2.
Work
Recall from previous physics courses, that when a force \( f \) acts over a distance \( d \) it does work, defined as
However, applied force does not have to be constant during a process. More generally, work is
where \( f \) varies along the path parameterized by \( x \) from position \( d_1 \) to position \( d_2 \), where we are assuming \( f \) is tangent to the path.
\(pV\) work
Work done by or on a gas, is known as \( \boldsymbol{pV} \)~work. A gas, such as the one enclosed in the cylindrical device in Figure 3, applies a force uniformly over an area \( A \), due to collisions against the wall, which is defined as pressure,
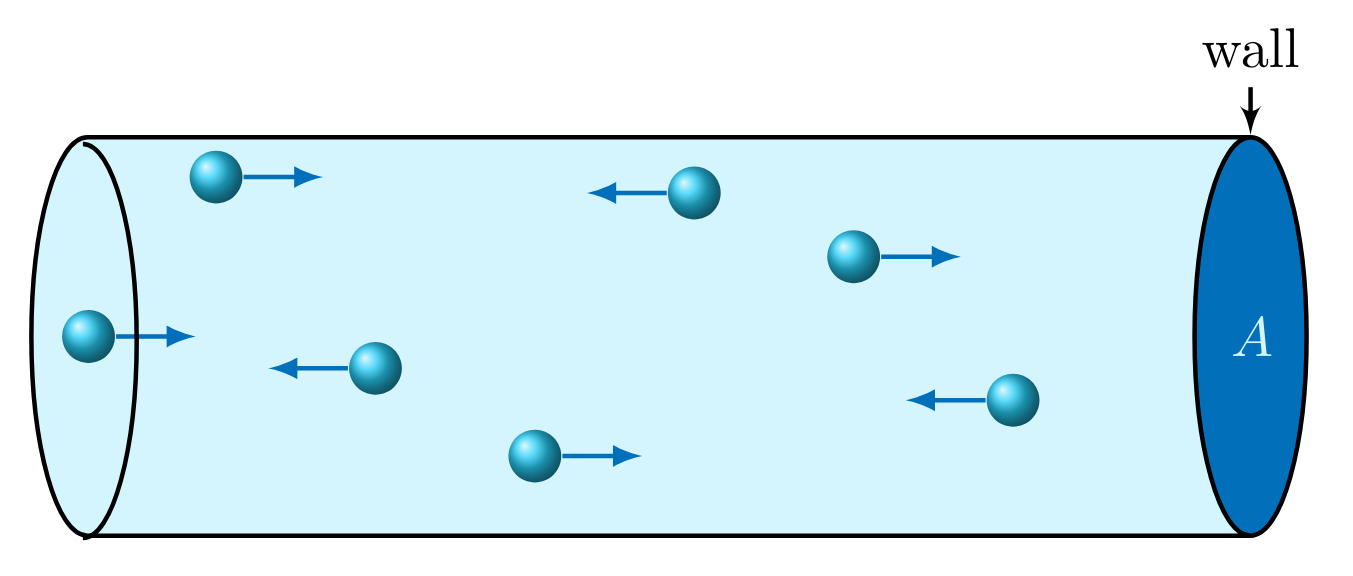
Referring back to the definition of work in Eqn. GenW, if \( x \) is a single cartesian direction normal to an area \( A \) then \( A\cdot dx \) is a differential volume \( dV \). Additionally, using the definition of pressure Eqn. pdefn, we can define \( pV \) work as follows,
\( pV \) diagrams
Graphs whose \( y \) and \( x \) axes are \( p \) and \( V \), respectively. According to the pV work equation, work is the area under the process curve. An example is shown in Figure 4.
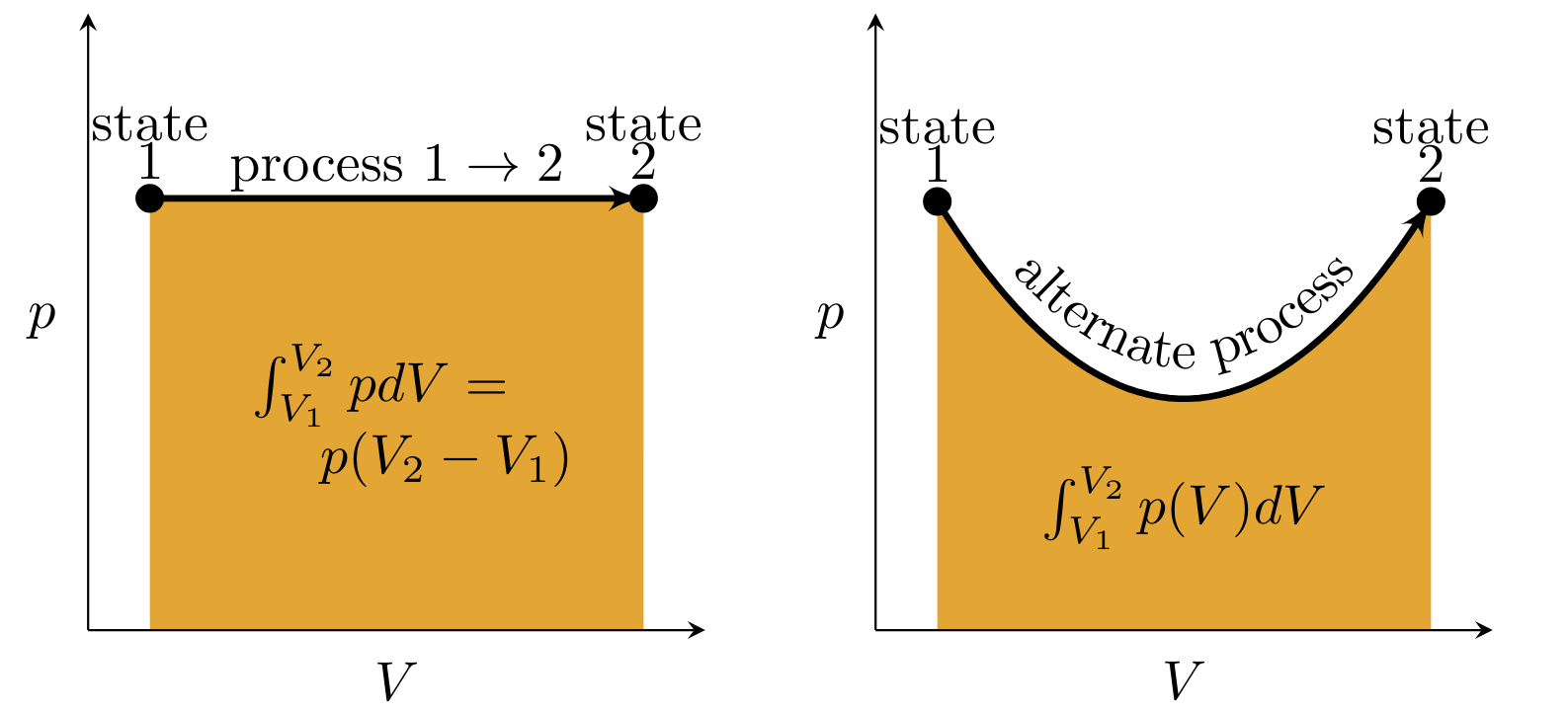
Isobaric Process:
During the process to go from 1 state to another, the system remains at a constant pressure, as shown in Figure 4 (left).
Work is path dependent:
Work transfer depends on the process. Figure 4 shows two processes where despite having the same initial and final thermodynamic states, the difference in magnitude of work for these two processes is different.
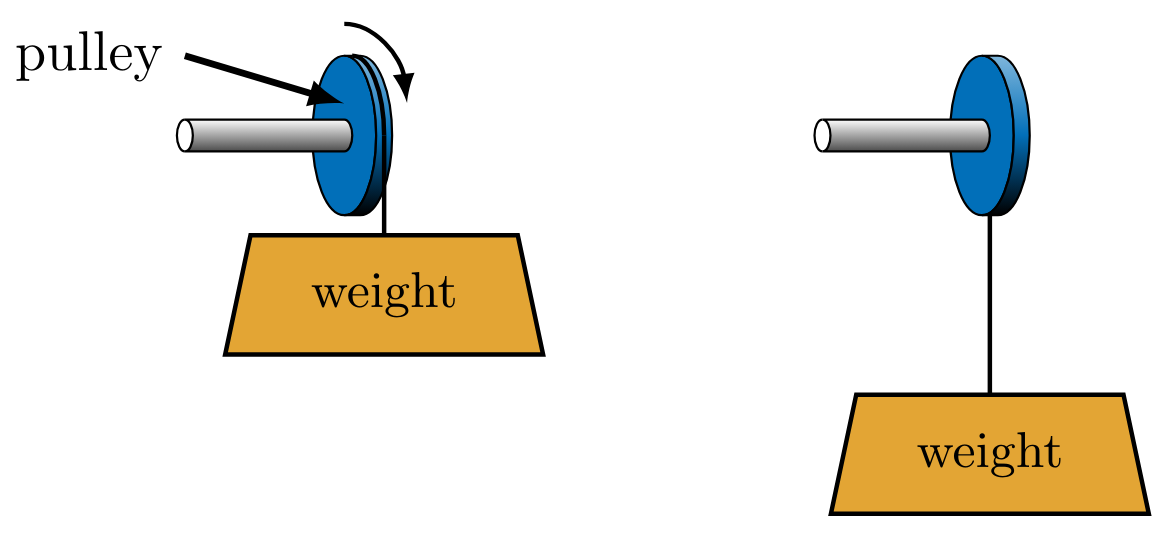
Shaft work
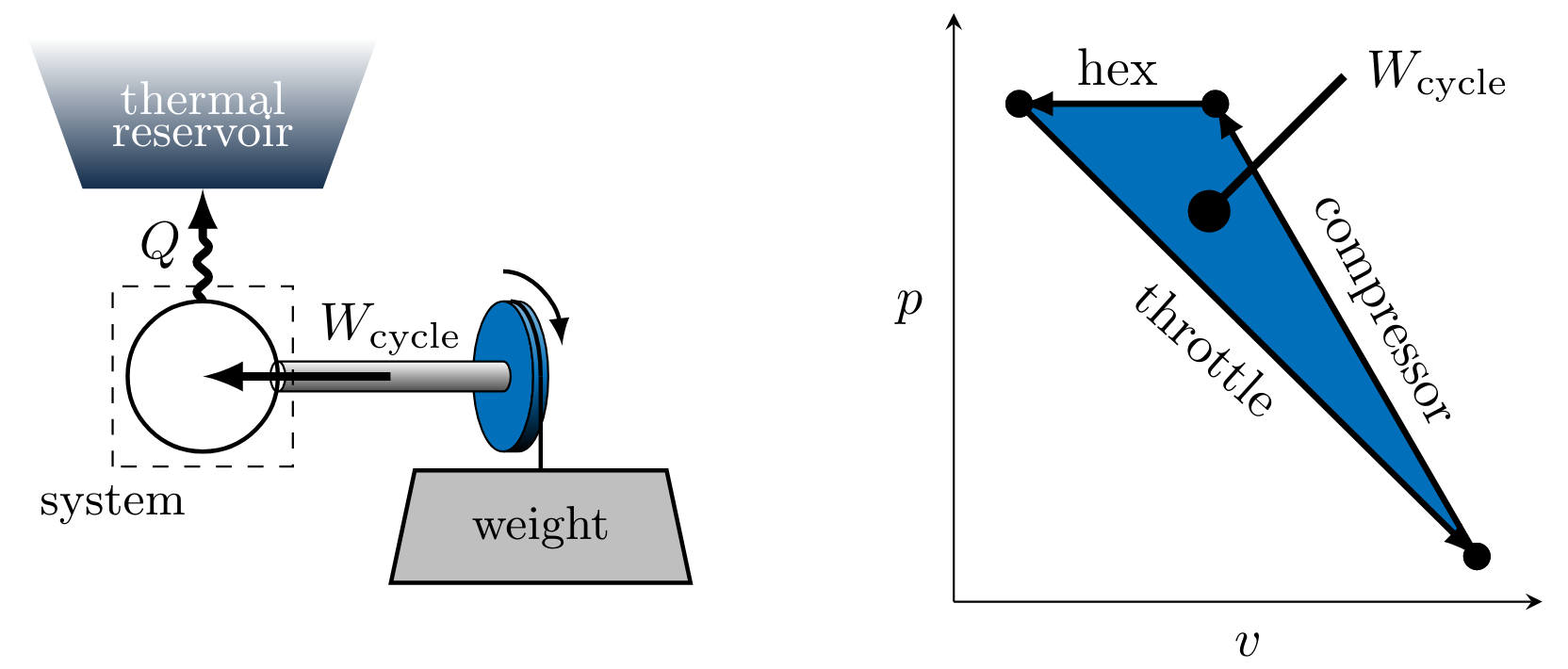
Work can also be done via shaft work. This can be explained by a shaft, on which a weight and pulley are mounted, providing a mechanism for inputting or extracting work as in Figure 6. Work \( W_{\text{cycle}} \) is done by the lowering mass on the system when the mass is released from its the raised position. The motion of the molecules in the system increases due to the shaft work input. This additional molecular energy temporarily increases the temperature of the system before the excess thermal energy is transmitted as heat \( Q \) to the reservoir.