Power Cycles
Much of the power that we use in our daily lives is produced through cycles operating with a working fluid.
Carnot cycle
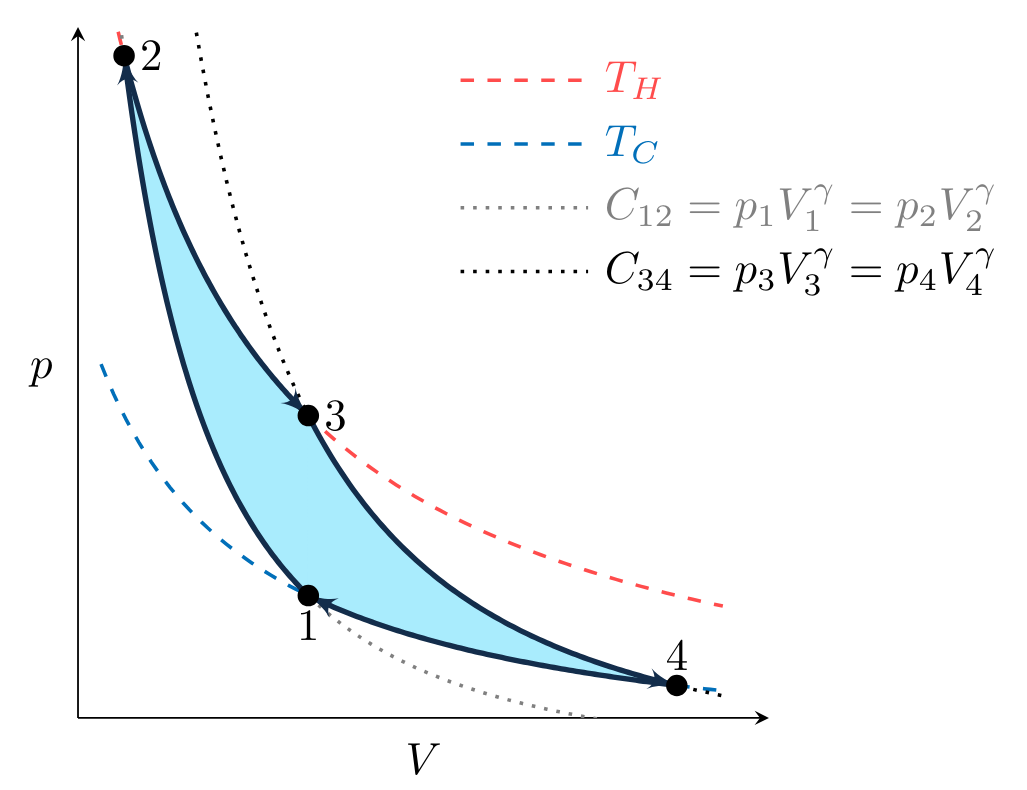
A Carnot cycle is defined as an ideal gas, undergoing a 4-step, reversible, closed thermodynamic cycle. Imagine that an ideal gas contained within a piston-cylinder undergoes the series of processes depicted in Fig. CarnotCycle below, with the gas starting in state
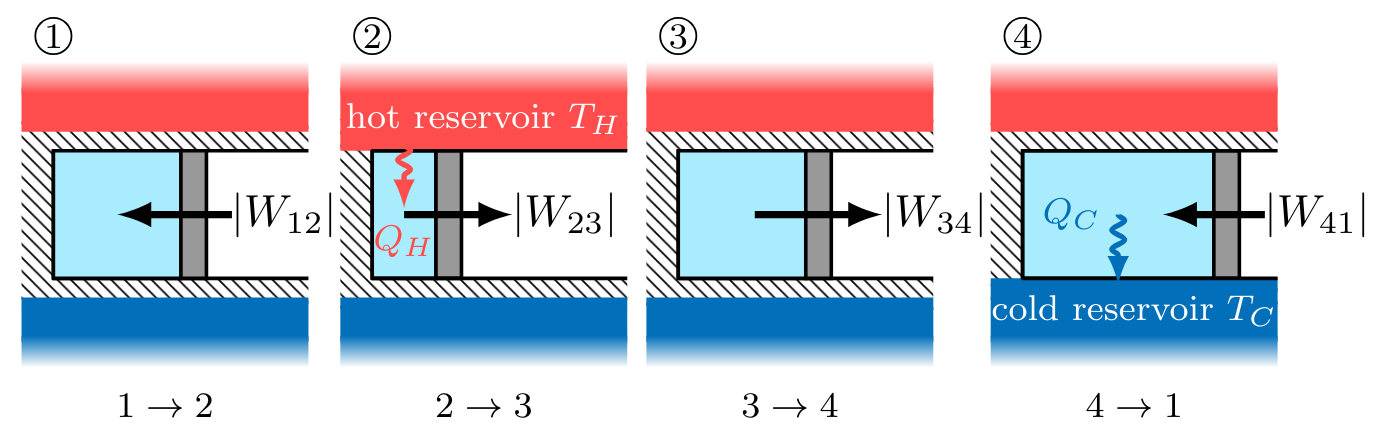
During the cycle, the system contacts sequentially a hot reservoir and a cold reservoir at temperatures
Reversible process:
In a reversible process, the direction can be reversed at any point by an infinitesimal change in external conditions.
Quasi-static process:
A process that occurs at an infinitesimally slow rate so that the system is in a thermodynamic equilibrium at all times.
Carnot Cycle Steps
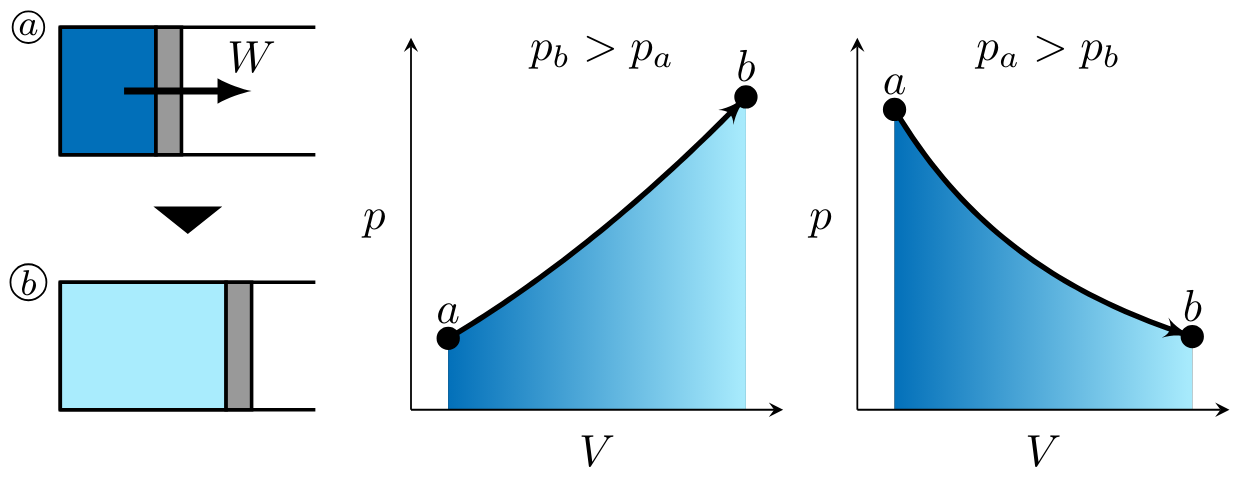
In processes
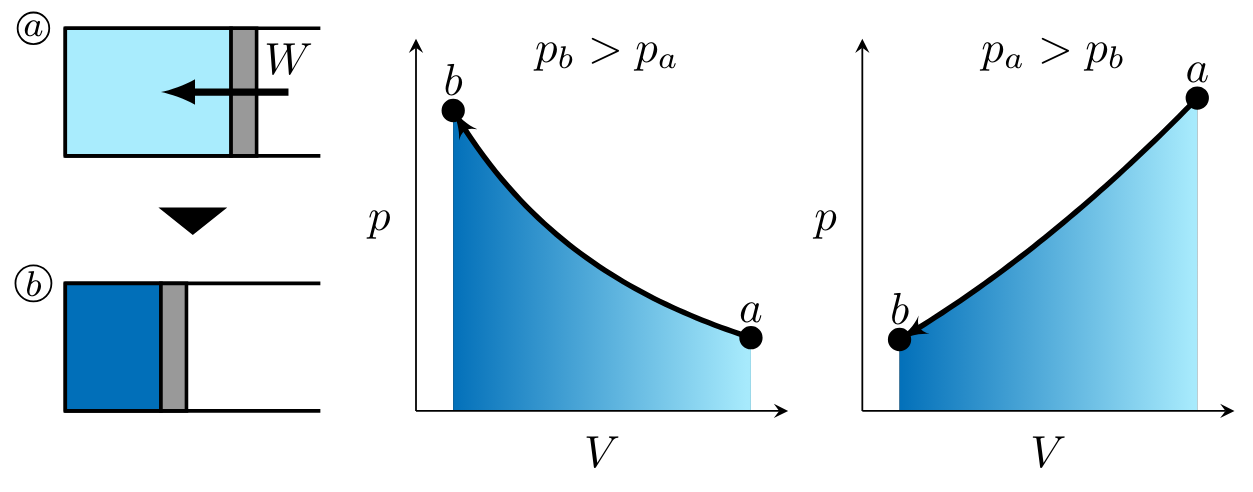
In processes
noting that work for these processes is negative as indicated by the right-to-left process curve arrow in each of the
To plot the processes that make up the cycle in Fig. CarnotCycle, we first observe that processes
where
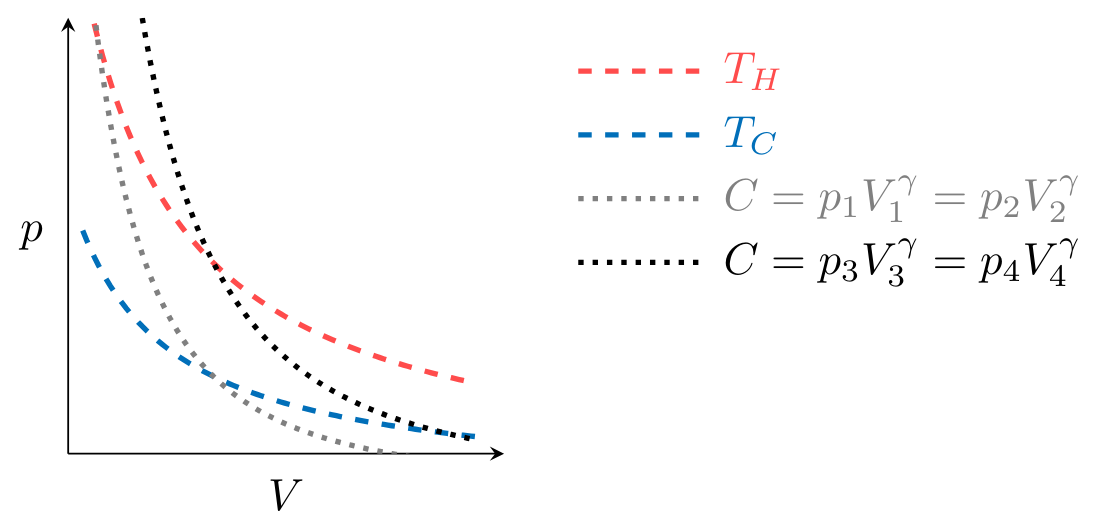
Processes
where
illustrated for two values of
Combining the
The work done by the cycle
Isothermal Processes
For isothermal processes
Because temperature is not changing and the fluid is an ideal gas, the change in internal energy for these processes is zero. An energy balance on the gas for each of these processes yields
where
where
Adiabatic Processes
For adiabatic, reversible processes
which, when integrated yield
Multiplying both sides of the relation in Eqn. polytropic by
It is interesting to determine the relationship between volume and temperature in the end states of processes
For an ideal gas,
Separating the volume
Integrating between states
The left hand side of both Eqns. VT12 and VT43 are equal so that
or equivalently\sidenote
Recalling the results from the energy balances on the isothermal processes (Eqns. QH and QC) we can show that the ratio of the heat influx
Applying Eqn. V1234, which defines the relationship between the states before and after the adiabatic processes, we obtain
for an ideal gas undergoing a Carnot cycle.
Internal Energy Balance of the System
The total change in the internal energy of the Carnot cycle
Thus the Carnot cycle is consistent with the energy change for a cycle in general.
Thermal Efficiency
This Carnot cycle turns heat input
Combining
A Carnot cycle produces the maximum possible efficiency for any heat engine. The efficiency can only approach
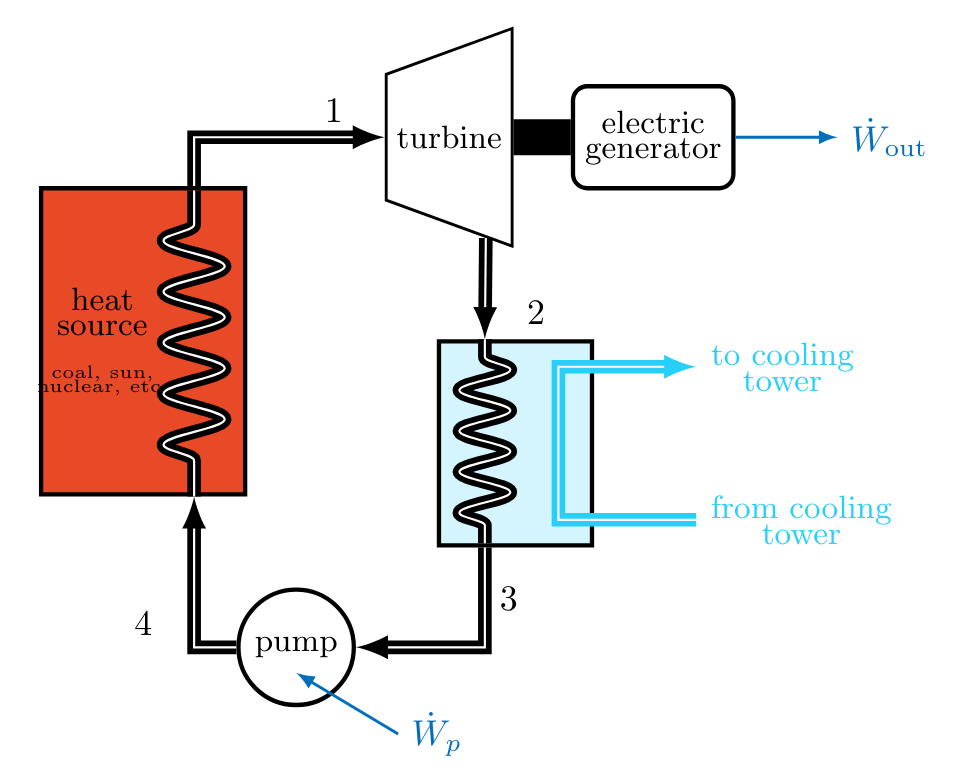
A Rankine Cycle
The Rankine cycle is the basis for steam-electric power plants, which produce nearly 90% of all electricity worldwide. This cycle includes two isentropic processes, and utilizes isobaric heat transfers.
An ideal Rankine cycle consists of the following internally reversible processes:
- Process
- Process
- Process
- Process
Instead, the condenser stream is taken to the saturated liquid state, so that only a compressed liquid must be pumped.\sidenote Cavitation is less of an issue in the two phase fluid used in the turbine since the vapor bubbles that form nearer the saturated vapor portion of the vapor dome are less driven to collapse. While state
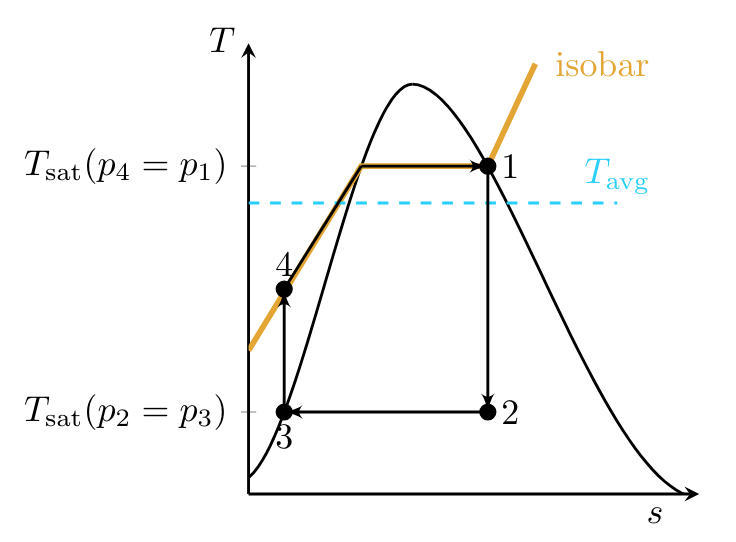
For reversible processes, the second law can be written as:
meaning that the area under the curve in a
Since the Carnot cycle tells us that the maximum thermal efficiency of a power cycle is:
it is clear from Fig. TsDiagramRankine that a hypothetical Carnot cycle with maximum operating temperature
Otto Cycle
The Otto cycle provides an approximation of the internal combustion engines that still make up the largest share of the transportation industry.
An automotive internal combustion engine uses a reciprocating piston-cylinder action to produce work. In a four-stroke engine
- The intake valve is open and the piston makes an intake stroke to draw in a fresh charge, e.g., a combustible mixture of fuel and air.
- The intake valve closes and the piston undergoes a compression stroke that increases the pressure and temperature of the air within. At the end of the compression, combustion is induced, e.g., via a spark plug.
- A power stroke follows as the gas mixture expands, doing work on the piston.
- Finally, the exhaust valve is opened and burned gases are purged during an exhaust stroke.
The Otto cycle simplifies this process by ignoring affects associated with the addition and removal of fuel. Instead it uses air, acting as an ideal gas, to provide insight into how such cycles can be optimized. The combustion itself is replaced by heat transfer and all processes are internally reversible. As a result, the Otto cycle consists of the following steps.
- Process 1-2: Isentropic compression of the air as the piston moves from bottom to top.
- Process 2-3: Constant-volume heat transfer to the air from an external source while the piston is at the top. (Approximates fuel ignition and rapid burning.)
- Process 3-4: Isentropic expansion (power stroke).
- Process 4-1: Constant-volume process in which heat is rejected from the air while the piston is at the bottom.
Note that because it is operating within a piston cylinder, the states the processes operate between are the state of the air in the closed piston cylinder system. This is different from the Rankine cycle, in which the stream states change by going through processes facilitated by different thermodynamics devices. We will show in class that the thermal efficiency of an Otto cycle can be expressed entirely as a function of the compression ratio,
and shows that engine efficiency can be increased by inducing a larger change in piston chamber volume. Of course, further practical considerations, such as the increased weight of such an engine, provide other design constraints. We only touch on a few of these design constraints, but in general thermodynamics provides many of the key principles through which energy systems can be optimized.